Let’s dive in and get technical!
Everyone has two kidneys which each have about 1.3 million nephrons. A nephron can be thought of as a filter unit where blood is filtered and anything deemed to be unneeded is discarded into the bladder to be expelled.
Nephrons contain feedback controls that finetune the filtration and enact changes to filtration criteria depending the body’s current needs and pressures. The details on this are a bit murky for me and thus I will not be discussing how this works. In addition, understanding these finetuning mechanisms is not needed to understand Alport Syndrome.
Each nephron, in simplistic terms, contains a blood vessel that brings in unfiltered blood, a filtration structure called the glomerulus, a blood vessel that returns the filtered blood back to the body, and a tubule that carries away the filtered waste from the blood. See figure below. [Fun fact tangent! The prefix “nephro” seen in medical terms involving the kidney comes from the Greek word for kidney “nephros”. Also, the word “glomerulus” is derived from the Greek word for ball “glomus”. A surgical procedure to remove a kidney or part of a kidney is called a nephrectomy. I guess it makes more sense to use the Greek root for this word instead of the English word as having a kidnectomy kind of sounds like you are eliminating a child.]
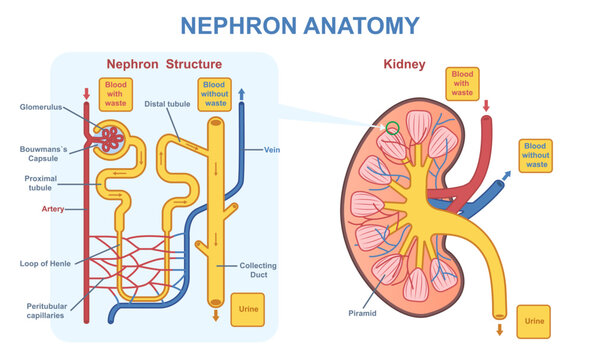
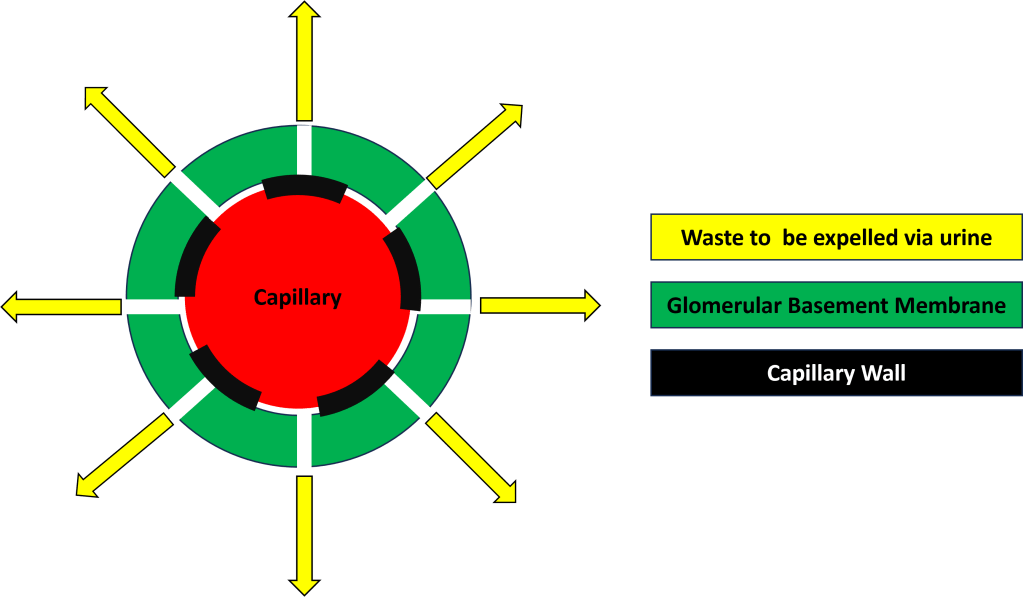
The glomerulus contains some complicated mechanisms and structures but essentially filtration occurs between the capillaries and the glomerular basement membrane (GBM). Fluids and waste filter out of the capillaries and through the GBM and end up being expelled via urine (Figure 2).
Now that we covered the kidney, let’s talk collagen or more specifically, type IV collagen, before going any further.
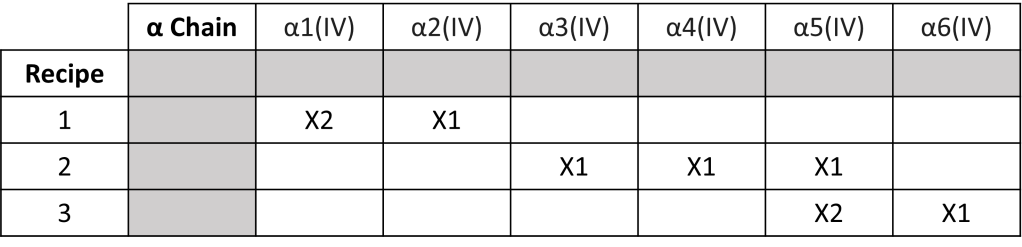
Type IV collagen is the main component of basement membranes found in the body. Type IV collagen molecules are constructed from six isomeric protein chains denoted by α1(IV) to α6(IV) [Chain isomers have the same chemical formula but differ in how their carbon atoms join together (for an analogy think of sandwiches where the ingredients are the same but the order in which they were put on the sandwiches differs).] There exists three kinds of type IV collagen. The recipe for each kind can be seen in the table below. You can see though that each type IV collagen molecule only uses three of the α chains in its construction.
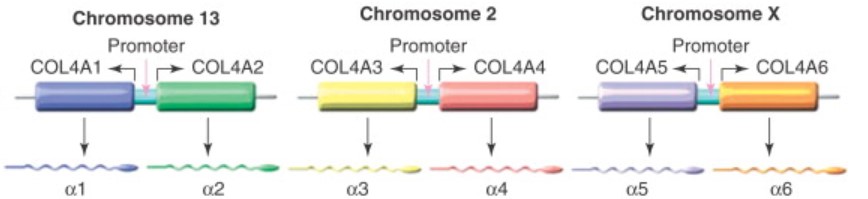
Each α protein chain is encoded by a specific gene found either on chromosome 13, chromosome 2, or the X-chromosome. The gene COL4A1 encodes for the α1(IV) chain, COL4A2 encodes for α2(IV), and so on with COL4A6 encoding α6(IV) (Fig. 3)
Alports is a genetic condition caused by mutations of the COL4A3, COL4A4, COL4A5, and/or COL4A6 genes. COL4A3 and COL4A4 are located on chromosome 2 and as such are involved in the autosomal inheritance (recessive or dominant) of alports. The genes COL4A5 and COL4A6 are located on the X chromosome and as such are X-linked in their inheritance.

The type of alport syndrome I have is X-linked. X-linked alport syndrome (XLAS) can be caused by a defect in only gene COL4A5 or a contiguous gene defect which involves the loss of genetic material and function of adjacent genes. Having only an issue with the gene COL4A5 is the most common type of XLAS. Having a contiguous gene defect usually involves the gene COL4A6 and potentially other nearby genes.
The type of XLAS I have involves a deletion of genetic material on COL4A5 and a deletion of uncertain significance on COL4A6. Having mutations in both the COL4A5 and COL4A6 gene, according to literature, appears to predispose one to experience diffuse leiomyomatosis (smooth muscle tumor growth); however, the gene defect in COL4A6 is still considered to be of unknown significance given the available data regarding Alport syndrome at this time.
Now back to the glomerulus and more specifically, the glomerular basement membrane. The kind of type IV collagen found in the GBM is that of recipe 2. It contains one α3(IV), α4(IV), and α5(IV) protein chain. Given the type of XLAS I have, deletion in COL4A5, my body is missing part of the instructions of how to make α5(IV) and α6(IV) protein chains. The effect of this is poor quality type IV collagen found in the GBM.
Sub par GBM type IV collagen results in the GBM in the glomeruli of all my nephrons to be weakened and prone to let larger molecules through such as protein and even red blood cells. This causes scaring and fibrosis of the GBM (think of a coffee filter where you keep trying to patch holes that appear. Eventually the filter wont filter due to there being too many holes or too much repair work will yield a filter in name only as it will no longer function.) See the representation of the damaged GBM at end stage kidney failure in Figure 5 and compare this visualization to healthy filtration in Figure 2.
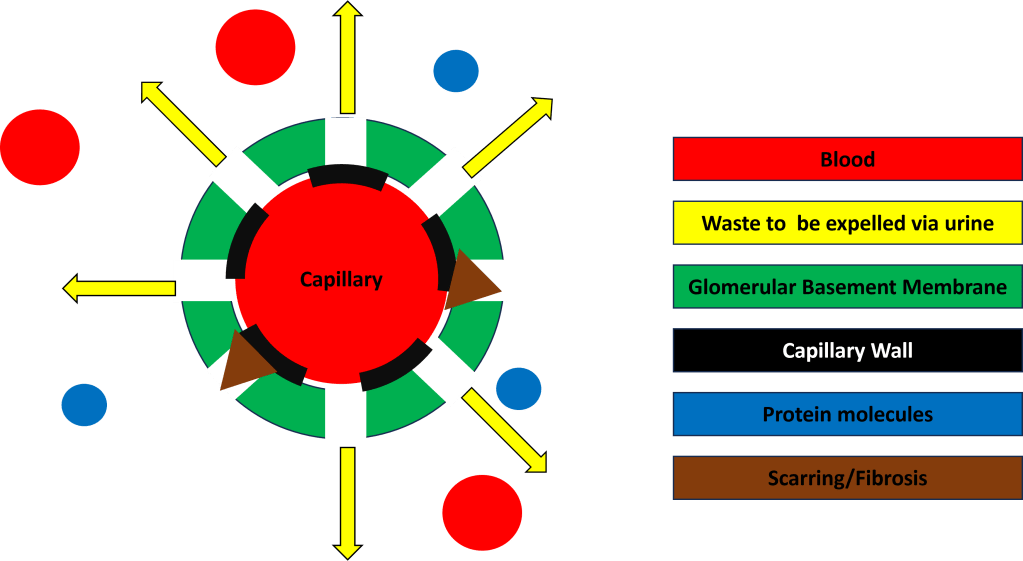
This slow damage of the GBM leaking protein and blood into my urine is what has caused the predicted failure of my kidneys. There is no cure for alports today as there is no way outside of sci-fi medical techniques to repair defective genes.